Is vaping really safer than smoking?
Many smokers are turning to e-cigarettes, but these products still produce chemicals that are hazardous to health

Exam links
This article links to the following topics in the AQA, Edexcel, OCR, WJEC, CCEA and SQA exam specifications:
• carbonyl compounds, aldehydes and ketones
• chiral molecules
• DNA
It is universally accepted that smoking cigarettes is bad for your health. As a consequence, many smokers have switched to using e-cigarettes to get their nicotine fix. But should we now be worried about other chemicals being inhaled as people vape?
The first smokers
The first Europeans to arrive in America at the end of the fifteenth century soon became acquainted with the Native American custom of smoking tobacco. They introduced the practice to Europe, and tobacco came to China and Japan at much the same time. In China, tobacco was taken up as a protection against malaria — its smoke was thought to repel mosquitoes. Many rulers were opposed to it — King James I wrote a book entitled A Counterblaste to Tobacco. But instead of banning it, governments settled for taxing it.
The compound responsible for the pleasurable aspects of smoking tobacco (and for its addictive properties) is nicotine, a secondary metabolite produced by the tobacco plant Nicotiana tabacum as a defence against herbivores. Other molecules used by plants as a defence include:
■ codeine and morphine in Papaver somniferum, the opium poppy (see CHEMISTRY REVIEW, Vol. 24, No. 2, pp. 31–33 and Vol. 27, No. 1, pp. 28–31)
■ caffeine in Camellia sinensis (tea) and Coffea spp. (coffee) (see CHEMISTRY REVIEW, Vol. 21, No. 1, pp. 10–15 and Vol. 24, No. 3, p. 34)
■ cocaine in Erythroxylum coca, the coca plant (see CHEMISTRY REVIEW, Vol. 27, No. 2, pp. 2–5)
A few insects are resistant to nicotine, particularly Manduca sexta (the tobacco hornworm) and Lasioderma serricorne (the cigarette beetle).
An accidental overdose
At one time nicotine was widely used as an insecticide. But nicotine is not just a fast-acting poison that is toxic to insects, it is toxic to humans too.
In 1932, some nicotine-containing insecticide was spilt on a chair in a Florida florist’s shop. The florist sat on the chair, the insecticide soaked though his trousers and nicotine was absorbed through his skin. The florist became unwell and was admitted to hospital for observation. The hospital did not know what was wrong with him, but as the body metabolised the nicotine (it has a half-life in the body of around an hour) his condition soon improved.
After a few days, the hospital discharged the florist, handing back his clothes, which had been kept in a paper bag while he was confined to bed. He put on his clothes again, and within an hour suffered a second dose of nicotine poisoning.
Small rewards
Smoking destroys most of the molecules present in tobacco, including nicotine. The nicotine that survives (around 10%) is carried from the lungs, via the blood, to the brain, where it stimulates release of neurotransmitters, including the ‘pleasure molecule’ dopamine (see CHEMISTRY REVIEW, Vol. 23, No. 4, pp. 12–13). The short time lag of less than 10 seconds between taking the ‘drag’ on the cigarette and the brain’s ‘pleasure’ response is the reason why smoking can be so addictive, as the brain links smoking with pleasure.
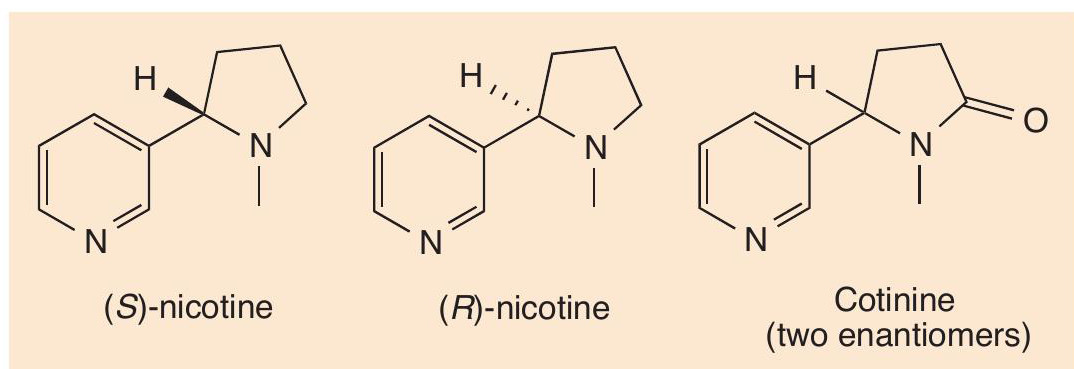
Nicotine contains a chiral carbon atom, so exists as two optical isomers (enantiomers, see CHEMISTRY REVIEW, Vol. 10, No. 3, pp. 6–9). As is usually the case with naturally produced chiral molecules, the tobacco plant makes just the one form, (S)-nicotine. The (R)-isomer is not made naturally, but can be synthesised in the laboratory (Figure 1).
The majority of the nicotine taken in by the body is oxidised in the liver to cotinine. Like nicotine, cotinine exists as (R)- and (S)-isomers. Cotinine is longer-lived than nicotine in the body, with a half-life of around 24 hours, so measures of cotinine levels in the blood or urine can be used to see whether someone has been smoking in the last day or two.



Big risks
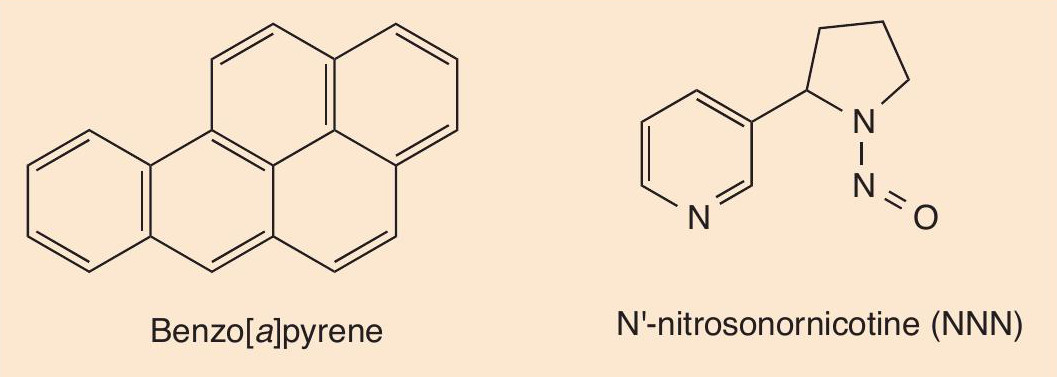
Most of the danger in smoking does not come directly from nicotine. Cigarette smoke contains around 4000 different compounds. The most dangerous molecules are carcinogens such asbenzo[a]pyrene and nitrosamines (e.g. N’-nitrosonornicotine, NNN, which is derived from nicotine). Tobacco ‘tar’, which gets carried in the smoke to the mouth and lungs, is rich in these toxic substances (Figure 2).
If you smoke tobacco, the smoke damages the DNA in the cells of organs exposed to it, as well as others that are indirectly exposed, speeding up genetic mutations and increasing the risk of cancer. It is not just the lungs. Other areas of the body, such as the mouth, larynx, liver, cervix, oesophagus, pancreas, bladder and kidneys are all at risk from smoking-induced mutations. Not all these mutations lead to cancer, but the more of them there are, the more likely it is that cancer-causing mutations will occur. The ways in which many of these cancers arise at the molecular level is not yet understood.
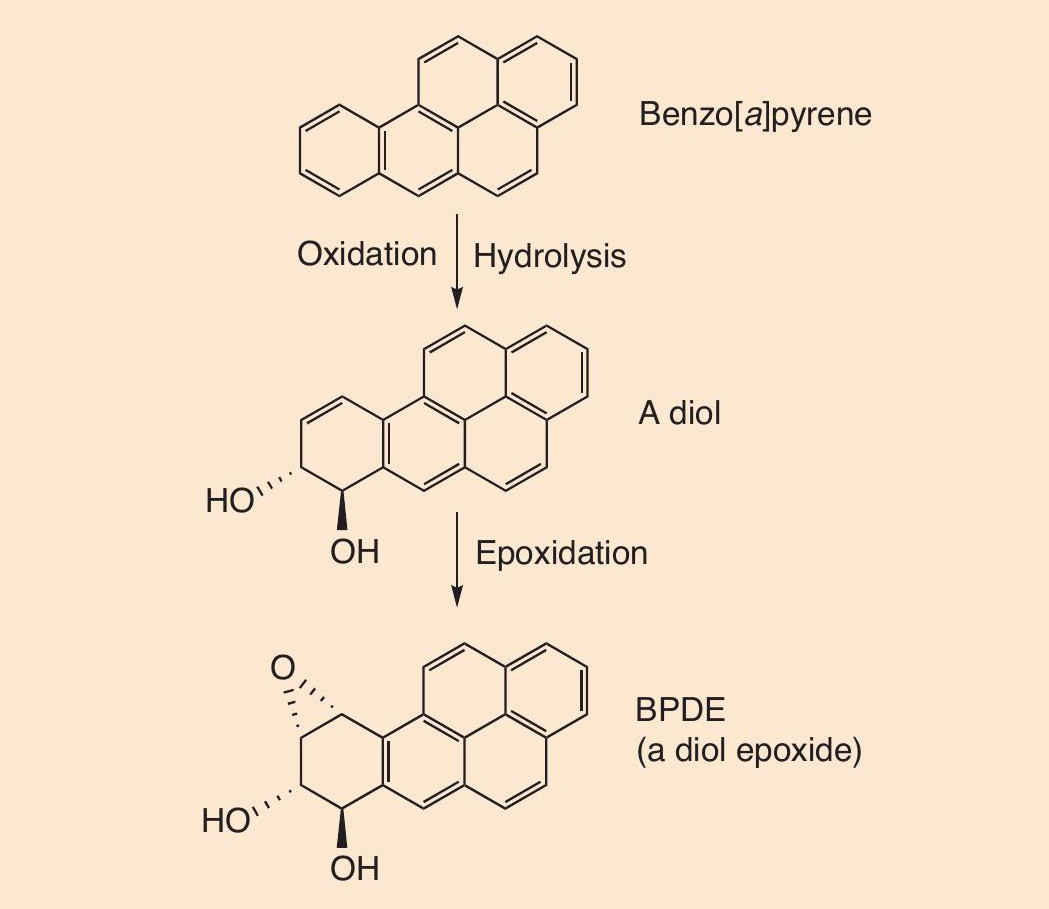
The polycyclic aromatic hydrocarbon (PAH, see CHEMISTRY REVIEW, Vol. 7, No. 2, p. 34) benzo[a]pyrene is present in tobacco smoke. Benzo[a]pyrene and other PAHs are well-known carcinogens. Once inhaled, PAHs get oxidised by cytochrome P450 enzymes to epoxides, which are rapidly hydrolysed to diols. The diols are next converted to diol epoxides, shown in Figure 3.
Benzopyrene diol epoxide (BPDE) reacts readily with DNA, principally at guanine, to form adducts (Figure 4) that lead to mutations (see CHEMISTRY REVIEW, Vol. 28, No. 4, pp. 2–6). These adducts deactivate tumour suppressor genes, leading to aberrant cells and starting the carcinogenic process.
According to the World Health Organization, smoking-related disease kills around 6 million people a year (about 600000 of these from ‘passive smoking’, see www.tinyurl.com/7pafwnz). Around 100000 of these deaths occur in the UK, and half a million in the USA. Nicotine does not cause these deaths directly, but addiction to nicotine does.
The rise of vaping
Within the last decade or so, e-cigarettes have been marketed as a means of taking nicotine with fewer health risks than traditional cigarettes. Since tobacco is not burned, e-cigarettes eliminate the risks associated with tar.

These e-cigarettes contain a battery, a metal heating element and a reservoir of e-liquid. The e-liquid is made up of:
■a solvent, either glycerol (propane-1,2,3-triol) or propylene glycol (propane-1,2-diol)
■ nicotine
■ flavourings
The e-liquid is converted to a vapour by the heating element and is inhaled by the smoker.
Hidden toxins
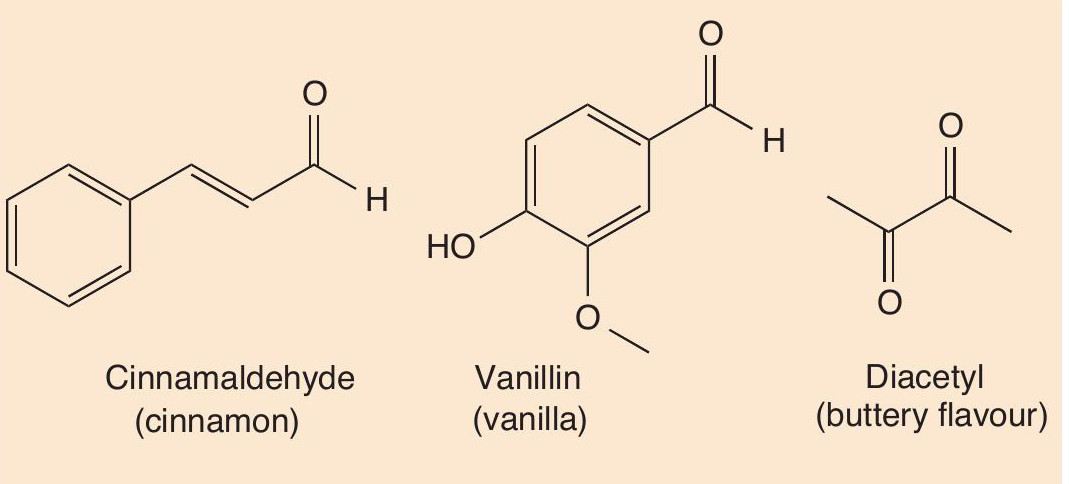
So what is the problem with e-cigarettes, if they do not produce carcinogenic tar? A significant number of the flavour chemicals contain carbonyl groups (aldehydes in particular) and these are often irritants of the mucosal tissue in the respiratory tract when inhaled. Typical examples of flavour molecules used are:
■ cinnamaldehyde (cinnamon)
■ vanillin (vanilla, see CHEMISTRY REVIEW, Vol. 28, No. 3, pp. 24–28)
■ diacetyl (which produces a buttery taste).
Diacetyl (butane-2,3-dione) is a ketone and is often used in artificial butter flavourings. It has been linked with bronchiolitis obliterans, a lung disease first observed in workers at a microwave popcorn factory in the USA. Tests show that a number of e-cigarette flavourings and their constituents (e.g. vanillin, cinnamaldehyde, diacetyl, isoamyl acetate and menthol, some of which are shown in Figure 5) may damage the linings of blood vessels and trigger future heart disease.
Although the flavourings used have been proven to be safe when used in food, that does not necessarily mean they are safe for other uses, such as in e-cigarettes. Studies have shown that some of these molecules, notably the solvents, can decompose when heated to above 300°C by the heating element in the e-cigarette.
Toxic aldehydes
Acrolein (propenal), methanal and ethanal (Figure 6) have come in for particular attention. These three substances, all aldehydes, are formed on decomposition of glycerol and propylene glycol.
Acrolein is the substance formed when cooking oil is heated until it begins to smoke — it is responsible for that ‘burnt fat’ smell. Acrolein is toxic and highly irritating to the eyes and nasal passages. Ethanal (acetaldehyde) and methanal (formaldehyde) are also toxic, with methanal in particular a well-known carcinogen.


These substances may be formed by decomposition of the flavouring molecules. For example, 2-methylbutanal, a flavouring with a chocolate smell, decomposes when heated to a number of products, including methanal and acrolein.
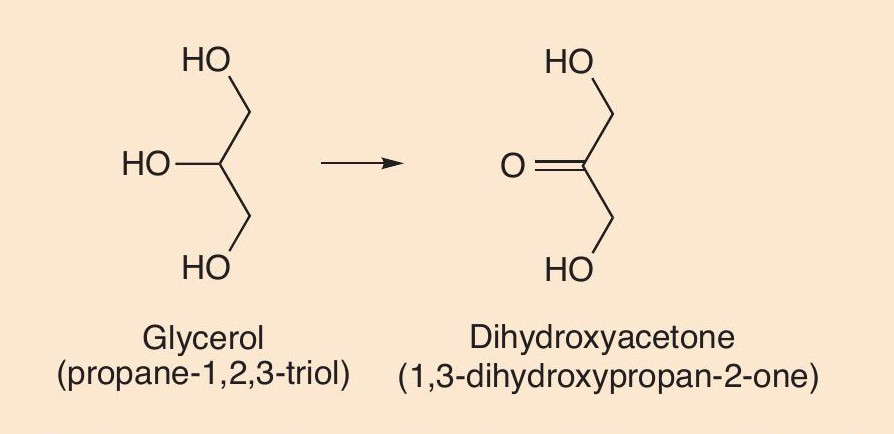
Another product of the thermal decomposition of glycerol in aerosolised e-liquid is dihydroxyacetone (1,3-dihydroxypropan-2-one), an ingredient of many spray tan lotions (Figure 7). It is known to have genotoxic and mutagenic properties.
How dangerous?
The use of e-cigarettes has been shown to lead to significantly lower levels of certain carcinogenic metabolites in the urine of users, compared to the levels found in smokers of traditional cigarettes. But e-cigarettes have also been linked with higher levels of some particulates in the aerosols they produce, including the metals nickel, silver and zinc. These metals are probably released from the heating element. The aerosols, containing metal particles, are taken into the lungs.
There is concern that the rapid growth in the use of e-cigarettes has not been accompanied by proper assessment of the risks accompanying their use, especially in the long term. While some reports claim that e-cigarettes are much safer than conventional cigarettes, one study has concluded that regular use of e-cigarettes by young people leads to them becoming heavier smokers of conventional cigarettes. Another study concluded that use of e-cigarettes by teenagers doubles the risk of coughs and bronchitis compared with non-smokers. While freely available to adults in the UK and the USA, e-cigarettes are banned or restricted in some countries, including Norway, Brazil, Singapore and Australia.
A letter published in 2015 in the British Medical Journal by Ricardo J. José summed up the current position:
‘Further basic science and epidemiological research is needed to increase our evidence base on the benefits and harms of e-cigarette vapour. Until then patients should not be misled into thinking that the likelihood of future harm is negligible when there is insufficient evidence to advocate this.’
At the moment, people who vape with e-cigarettes are guinea pigs, as nobody knows whether there are long-term risks to their health.
Key points
• The small amount of nicotine that survives when tobacco is burned is carried from the lungs, via the blood, to the brain, where it stimulates neurotransmitters associated with pleasure.
• Nicotine is not the most dangerous chemical in cigarette smoke, which contains roughly 4000 different compounds, including those that alter DNA and may lead to cancer.
• E-cigarettes do not contain tar, but introduce other molecules into the body, such as flavour chemicals, some of which are irritants.
• Users of e-cigarettes have significantly lower levels of certain carcinogenic metabolites in their urine, compared to the levels found in smokers of traditional cigarettes. But metals such as nickel, silver and zinc have been detected in e-cigarette vapours.
Glossary
Aldehyde A carbonyl compound with the C=O (carbonyl) group at the end of the carbon chain, also recognisable as a –CH=O group.
Aromatic Compounds where the π electrons are delocalised over a complete ring system, benzene being the simplest example.
Carbonyl group Functional group made up of a carbon atom and an oxygen atom linked by a double bond.
Carcinogen A substance capable of causing cancer.
Chiral carbon A carbon with four different groups bonded to it. The resulting molecule is not superimposable on its mirror image.
Diol An alcohol with two hydroxyl (–OH) groups per molecule.
Epoxide A cyclic ether containing a three-membered ring, one of the atoms in the ring being an oxygen and the other two carbons.
Genotoxic Agents that cause damage to the genetic material found within a cell.
Guanine A nitrogen-containing cyclic compound that is one of the four nucleotide bases, which form a constituent part of nucleic acids such as DNA.
Half-life The time it takes for one half of the original amount of a particular chemical to be used up.
Hydrocarbon Compound containing only carbon and hydrogen atoms.
Ketone A carbonyl compound with the C=O group in mid-chain.
Mutagenic Induces mutations in the genes of an organism (i.e. changes in the sequence of nucleotide bases in DNA).
Neurotransmitter A molecule that is released by nerve cells (neurones) and crosses the gap (synapse) between nerve endings, so transmitting signals (nerve impulses) between one nerve cell and the next.
Optical isomers (enantiomers) Two molecules with the same formula, containing identically connected atoms, which are mirror images of each other.
Polycyclic Molecule containing a number of rings fused together.
Secondary metabolite A compound in an organism, such as a plant, which is not responsible for its growth or reproduction, but that is beneficial in other ways, such as defence.
Practice exam questions
Use the three molecules shown in Figure 5 (cinnamaldehyde, vanillin and diacetyl) to answer the following questions:
1 Name the three functional groups found in the vanillin molecule. (3 marks)
2 Suggest a chemical that would give a positive test with all three molecules. Describe what would be seen. (2 marks)
3 Suggest a chemical test that would distinguish between diacetyl and the other two molecules. (2 marks)
ChemistryReviewExtras
Check your answers at www.hoddereducation.co.uk/chemistryreviewextras





