Oil on troubled waters

In historic adventure novels we hear tales of animal fat or oil being used to calm a stormy ocean (Box 1). But can oil really save a ship from wrecking?
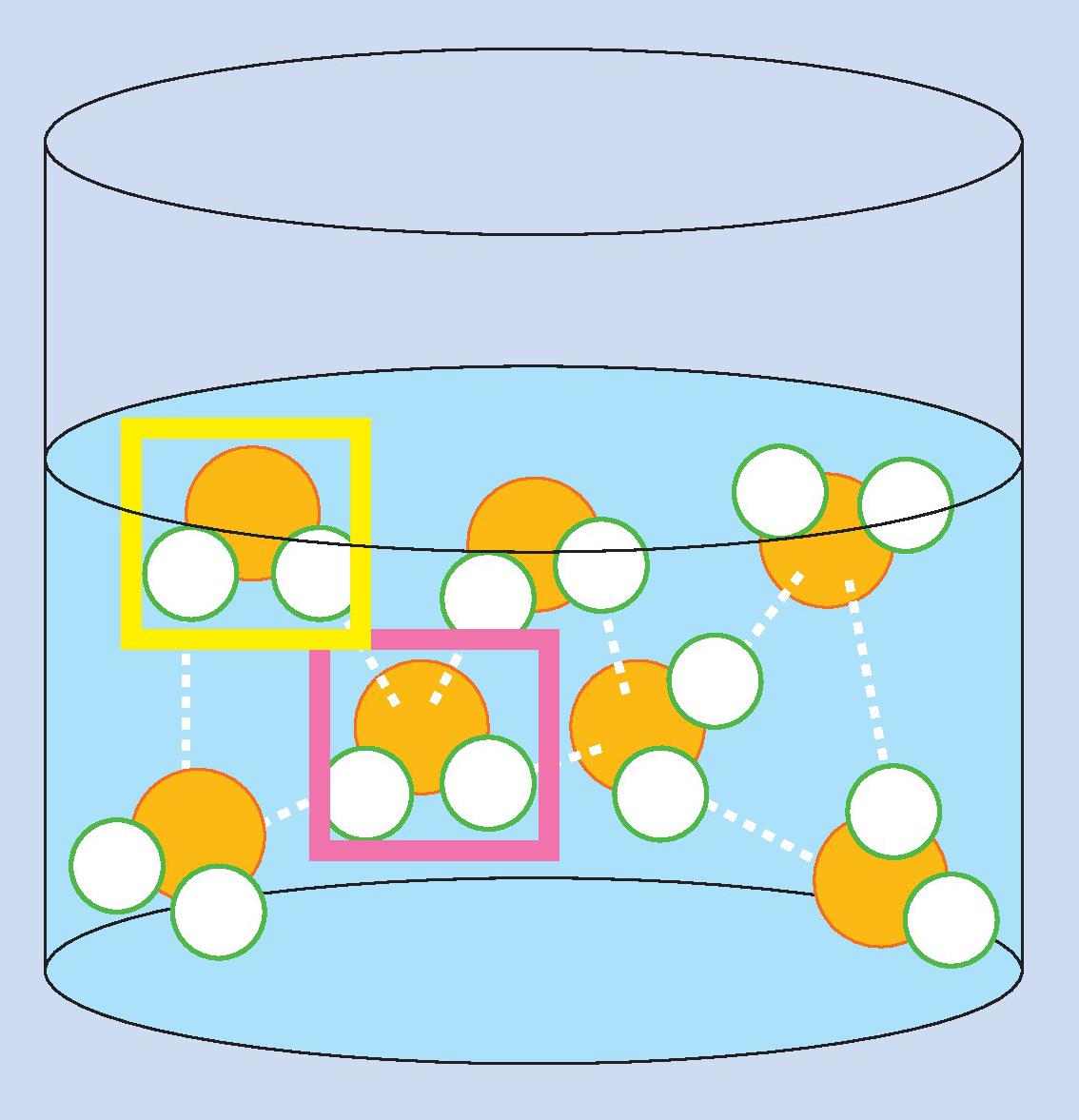
The extent to which the wind can generate waves depends on how good the grip is between the air and the water surface. As the wind blows over the sea, it applies a force across the surface, pushing the water. In water, hydrogen bonds form between oxygen and hydrogen atoms (Figure 1). Molecules on the surface (marked in yellow) have fewer neighbours with which to form bonds than those within the liquid (marked in pink). The stronger the forces between molecules, the more energy is required to move them to the surface.
Calming the waves
When the wind blows across the sea, it creates a ‘well’ in the water and the total surface area increases. The smaller the well, the fewer attractions will be lost between molecules. Therefore it requires less energy to bend the water surface as a swell than to break the surface as a storm wave.
Oil will spread out to form a thin layer on top of the water, just one oil molecule thick. Only the hydrophilic (water-loving) ‘heads’ of the oil molecules are in contact with the water. The long hydrocarbon ‘tails’ stick out of the water, as they are hydrophobic. Oil molecules have weaker intermolecular interactions than water molecules, and so have a lower surface tension.
Box 1 Sand and oil
‘Dick Sand, by a supreme precaution, had also brought on the forecastle ten barrels of the cargo containing whale’s oil. That oil, properly poured the moment the “Pilgrim” would be in the surf, ought to calm the sea for an instant, in lubricating, so to say, the molecules of water, and that operation would perhaps facilitate the ship’s passage between the reefs.
“Pour the oil — pour!” exclaimed Dick Sand. Under this oil, which was poured on it in quantities, the sea grew calm, as by enchantment, only to become more terrible again a moment after. The “Pilgrim” glided rapidly over those lubricated waters and headed straight for the shore.’
From Dick Sand, A Captain at Fifteen (Jules Verne, 1878)
Oil works to calm the stormy water because it is a lubricant, i.e. it reduces friction. When the wind blows over the oil on the water, it will be less efficient at pushing the water, as it slides over the surface more easily. As the effect of the wind on the water is reduced, the magnitude of the waves decreases, making it safer for ships.





