Weaponised insects
Insects use intricate chemistry to protect themselves from their enemies.

EXAM LINKS
This article links to the following topics in the AQA, Edexcel, OCR, WJEC, CCEA, SQA and IB Diploma exam specifications:
■ acid and base reactions
■ intermolecular forces and volatility
■ organic functional groups
Insects need to defend themselves from predators, especially as their small size makes them vulnerable. They have evolved various ways of doing this, and many insects have specialised defences that use a rich variety of chemicals. Some of these are toxic, while others have smells that act as deterrents. Insects employing such chemicals often have striking colours, warning of their defensive armoury, telling foes that they are dangerous or at least ‘not good to eat’. Such colouring is termed aposematic. Ladybirds are a familiar example of this, their colour scheme warning predators of their bitter taste.
Useful acids
Ants are possibly the insects best known for chemical defence. About 30% of ants belong to the Formicinae subfamily, which produce a venom containing up to 60% methanoic acid (HCOOH), traditionally called formic acid, a name derived from the Latin noun formica for ant. This is discharged as a spray from a sac in the abdomen. This formic acid has additional uses in some other formicine ants. Lasius neglectus workers use it on their brood to inhibit the growth of fungal pathogens, while Nylanderia fulva (the tawny crazy ant) workers use their venom to detoxify the alkaloids in the venom of Solenopsis invicta fire ants. S. invicta has a multicomponent venom, including toxic proteins as well as piperazines, such as 2-methyl-6-undecanylpiperidine (Figure 1), which causes intense burning pain when humans are stung (hence the name fire ants). It produces an allergic reaction in up to 25% of those stung, which can cause anaphylactic shock that sometimes proves fatal. Formic acid might denature enzymes present in the fire ant venom, as well as neutralising the basic piperazines.


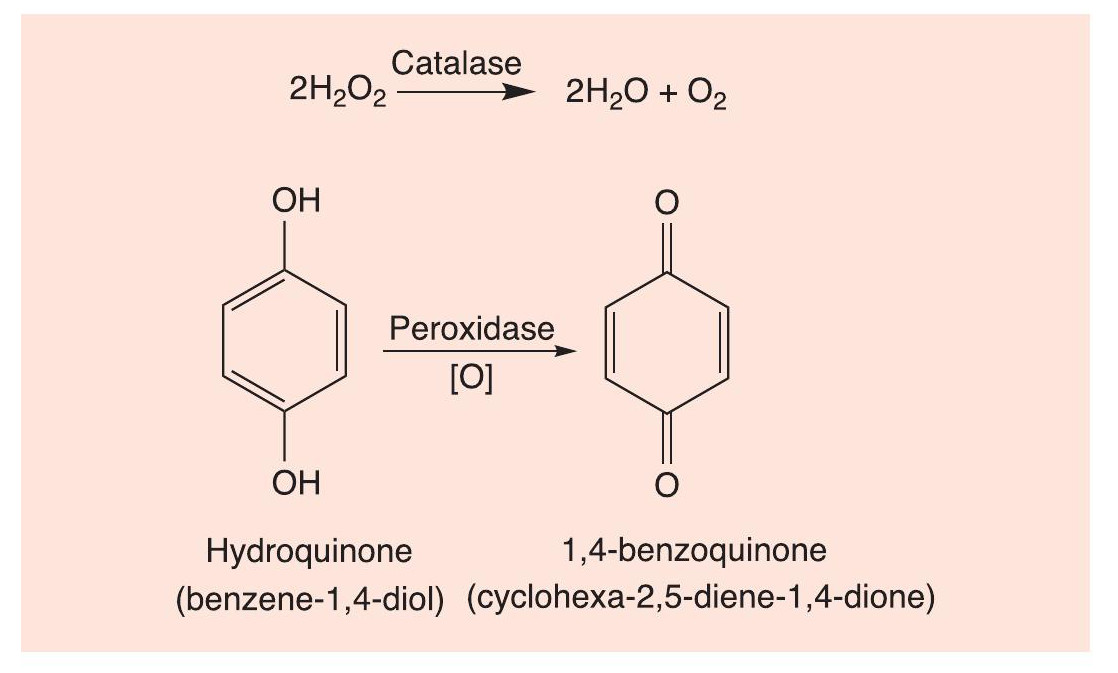
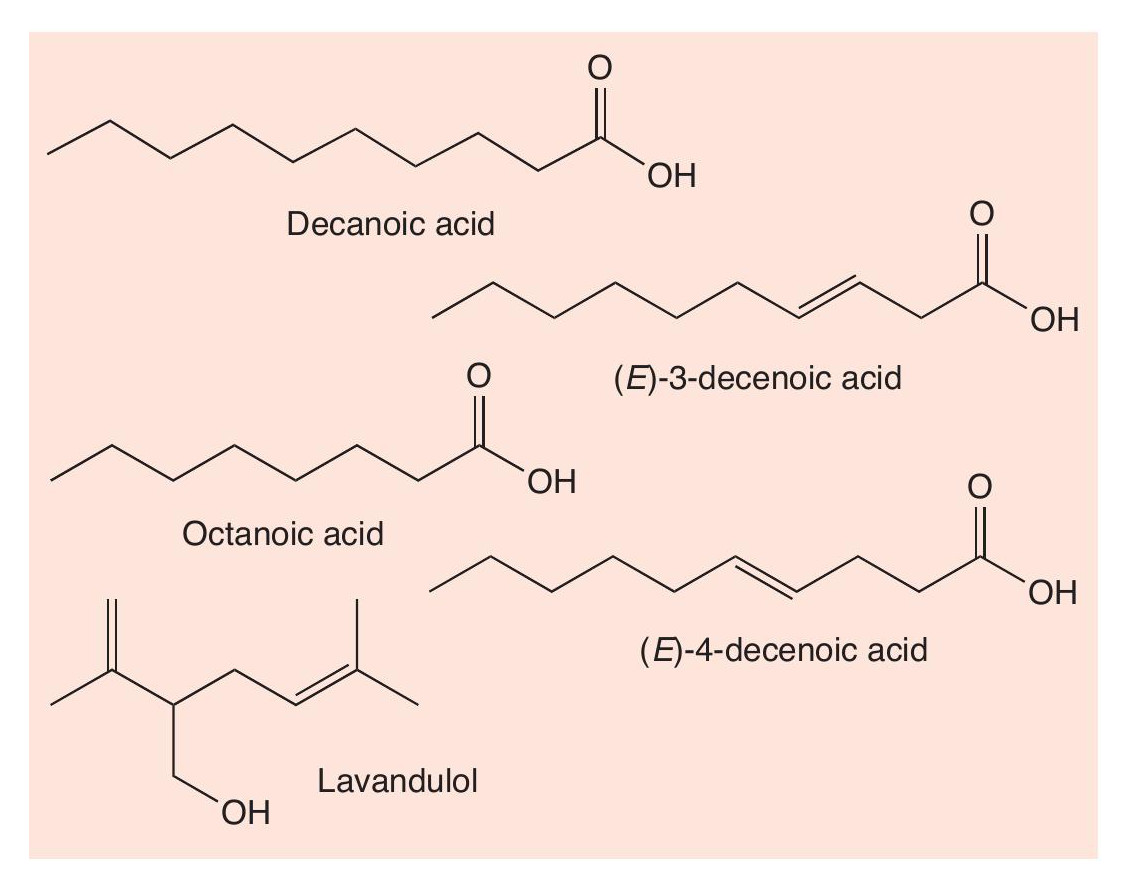

Hot shots
Perhaps the nearest approach to a pistol-packing insect is provided by the bombardier beetle (e.g. Stenaptinus insignis),which has been called ‘the insect world’s greatest marksman’. The insect separately synthesises hydrogen peroxide (H2O2) and hydroquinone (benzene-1,4-diol). When the insect is threatened, it delivers them to a reaction chamber whose walls produce enzymes (catalases and peroxidases). Catalase decomposes H2O2 to water and oxygen, while peroxidase oxidises hydroquinone to 1,4-benzoquinone (cyclohexa-2,5- diene-1,4-dione), the reaction producing temperatures up to 100°C (Figure 2). The hot mixture is propelled by the pressure of the oxygen gas out of the revolving tip of the abdomen, which acts as a ‘gun turret’, aiming the spray very accurately to its rear and either side at predators. The benzoquinone is very irritating to the prey’s eyes and respirator y system, possibly fatally. Understandably, the reaction chamber has a chemical resistant lining made of chitin, a tough polysaccharide.
The bombardier beetle is not the only spraying insect. Acids can be used against ants as well as by them. The red-lined carrion beetle (Necrodes surinamensis) uses a mixture of octanoic acid, decanoic acid, (E)-3-decenoic acid, (E)-4-decenoic acid and various terpenoids (Figure 3). One of these terpenoids is the unsaturated alcohol lavandulol, which is also an ingredient of lavender oil. The beetle discharges this mixture through a jet at the tip of the abdomen, which it can aim in any direction at attacking ants and birds.
Foul foam and special sprays
Eastern lubber grasshoppers (Romalea guttata) are brightly coloured — awarning of their biohazards. When threatened, R. guttata emits a foul-smelling and foul-tasting froth from its thorax. The froth has been shown to contain a corrosive cocktail of compounds (Figure 4). The frothy bubbles burst, creating an unpleasant mist.


The ‘twostriped walkingstick’ (Anisomorpha buprestoides) is the most common stick insect in Florida (USA). It produces a strongly smelling defensive substance, anisomorphal (Figure 5), which is irritating to humans, even when inhaled, and is very painful if in contact with the eyes. The insect sprays this secretion from two sac-like glands on its thorax, which have openings just behind its head. The walkingstick can aim up to 30–40 cm with accuracy, making its escape while its predator is having to clean off the spray.
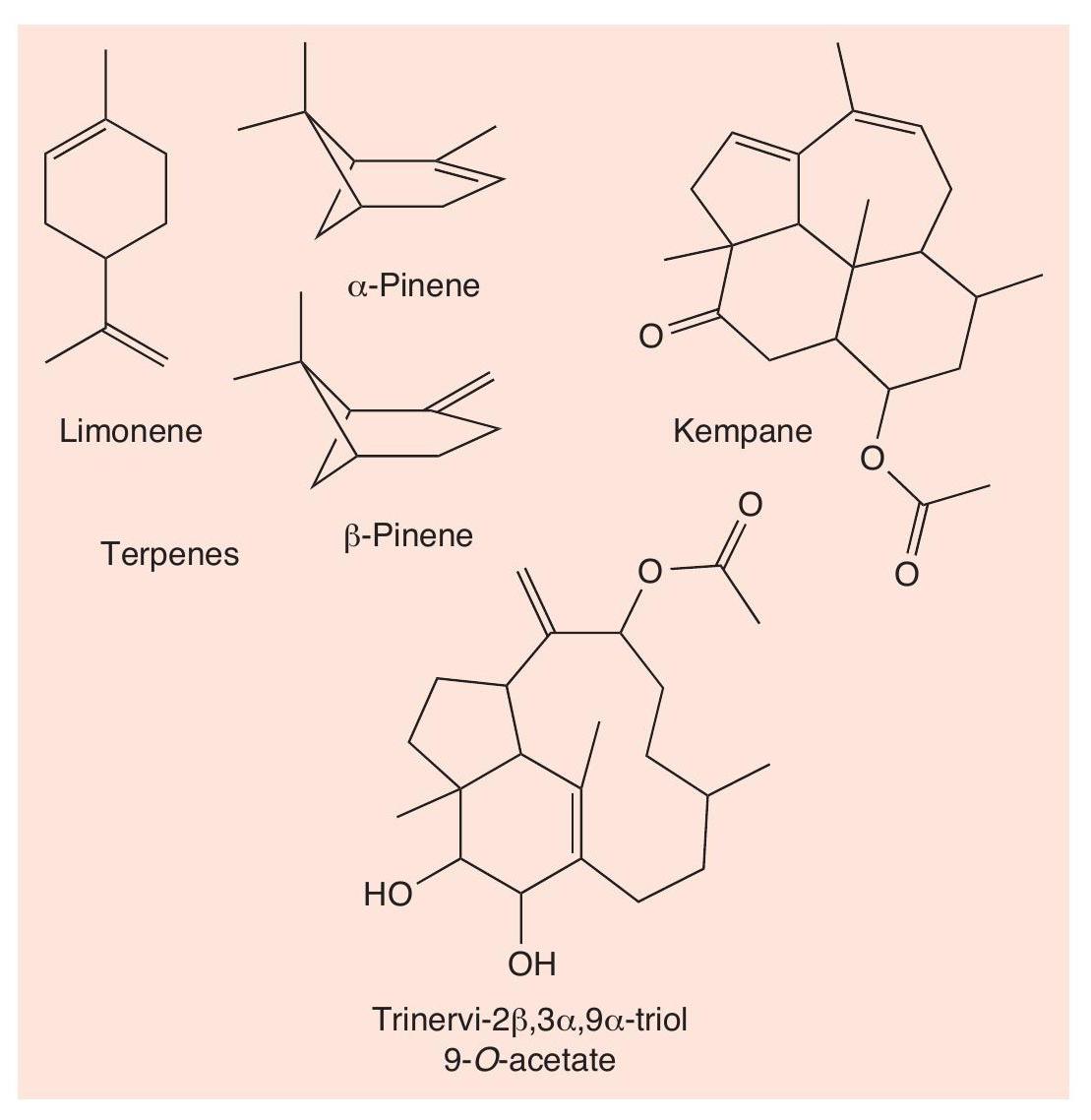
The soldiers of the Australian termite (Nasutitermes exitiosus) have a gland on their heads through which they discharge a sticky and odorous mixture as a fine filament, when disturbed or threatened — usually by ants that have entered their termite mound. The termites can discharge the spray with great precision ahead, to the sides and even behind. The mixture has two types of component (Figure 6). The smell is provided by familiar terpene hydrocarbons (CHEMISTRY REVIEW Vol. 26, No. 1, pp. 2–7), notably limonene and both α- and β-pinene, which act as irritants to the target. The stickiness is due to much rarer substances, including kempane and trinervi-2β,3α,9α-triol 9-O-acetate, which are much larger molecules and therefore much more viscous — tacky enough to stick the invading ants to the walls of the mound. The smell of the simple terpenes, which being small molecules are quite volatile, also acts as an alarm pheromone, alerting and recruiting other soldier termites who spring into action to encircle the prey. The termites do not only use their defensive spray against ants, but also against spiders, centipedes and other arthropods, to all of whom the chemical mixture proves fatal.
Deadly cyanide
Carbonyl compounds react with hydrogen cyanide (involving the CN–ion) to form cyanohydrins. Cyanohydrins are involved with defence in a number of insects and other arthropods. Soil centipedes of the family Geophilidae secrete a sticky substance containing hydrogen cyanide (HCN), which is very toxic, as it interferes with cytochrome oxidase enzymes, thus stopping respiration. The centipedes store the cyanohydrin addition products, mandelonitrile and benzoyl cyanide, which are broken down enzymatically into HCN and benzaldehyde and benzoic acid, respectively (Figure 7). Flat-backed millipedes of the order Polydesmida, such as Apheloria corrugata, have a similar defence of producing hydrogen cyanide.
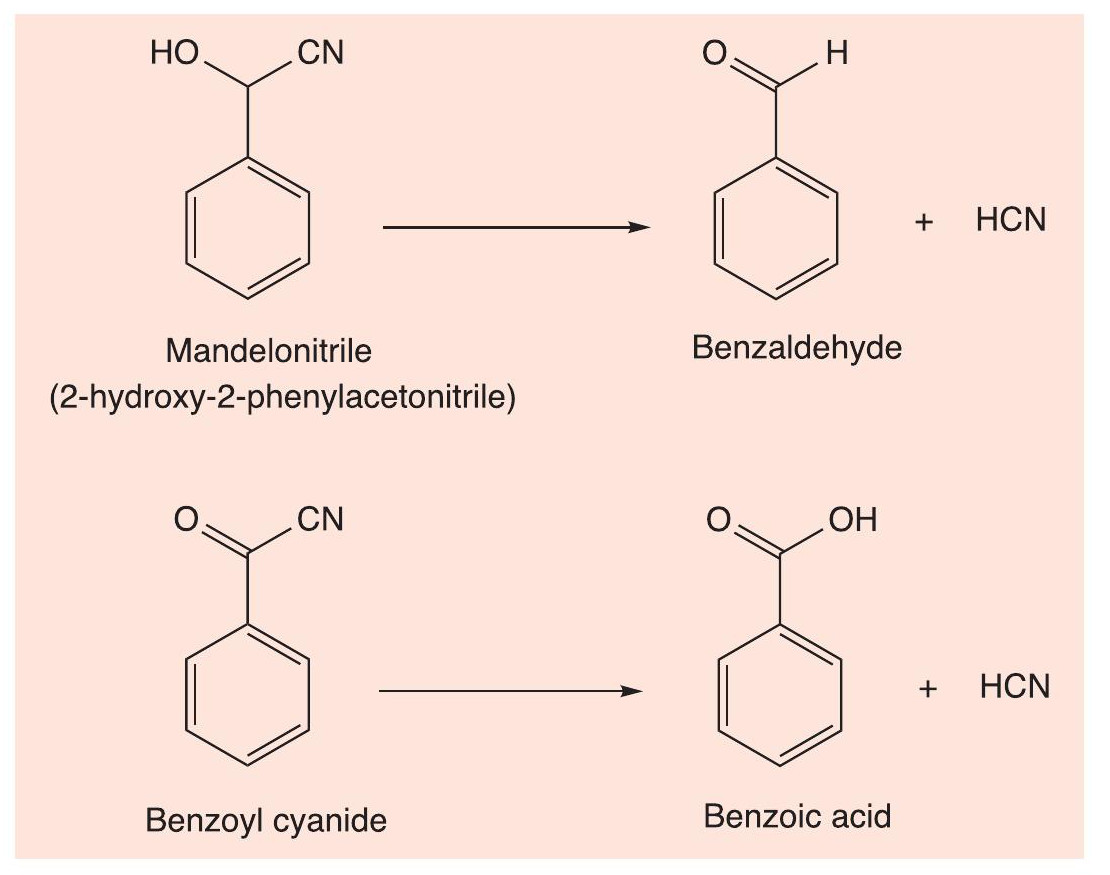
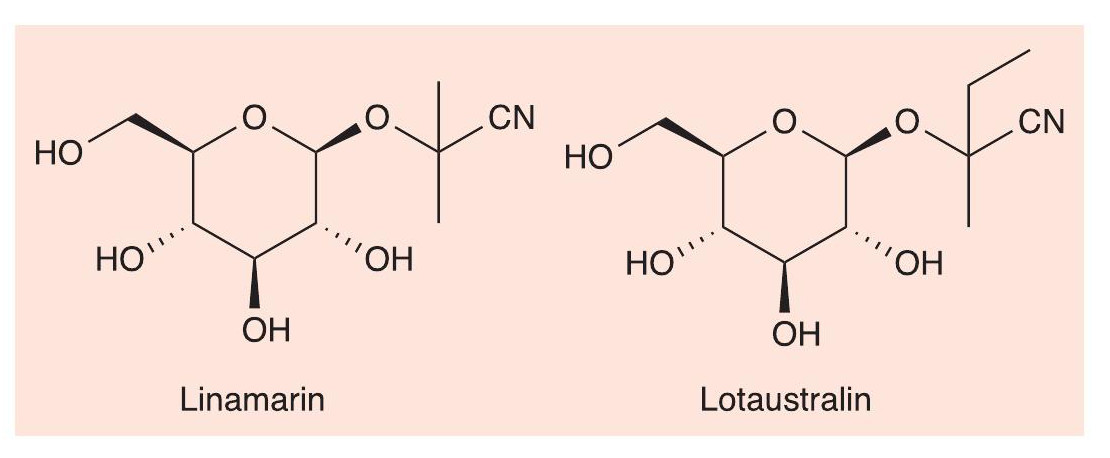

Some butterflies and moths also make use of HCN in defence. The six-spot burnet moth (Zygaena filipendulae) flaunts a striking black and red wing pattern, a classical example of aposematism, advertising its unpalatability to predators. This is due to the compounds linamarin and lotaustralin (Figure 8), where the cyanohydrins of propanone and butanone, respectively, are linked to glucose as glycosides. The moths synthesise these compounds themselves, as well as obtaining them from their larval host plant, bird’s-foot trefoil (Lotus corniculatus).
Blister beetles
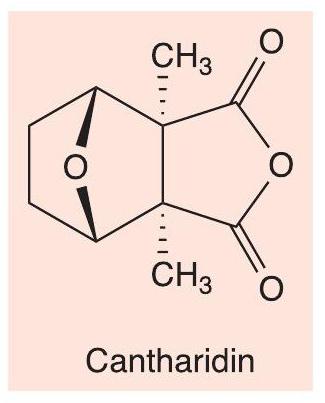
For a final example of toxic chemicals, let us look at cantharidin (Figure 9), which is found in blister beetles of the family Meloidae (see CHEMISTRY REVIEW Vol. 29, No. 1, pp. 18–21). Male beetles make cantharidin as a defensive agent, which is passed on to females in mating and used by them to protect their eggs, for example from ants. When disturbed, the insects ‘reflex bleed’ from their knee joints, and the blood contains cantharidin, which is very toxic, particularly to the renal and reproductive systems, with a lethal dose of the order of 100 mg in humans. If horses eat hay infested with these beetles, the horses can be poisoned.

Box 1 Volatility of molecules
How volatile a molecule is depends upon intermolecular forces and therefore how much energy is needed to vaporise it. All other factors being equal, this depends upon molecular size, as van der Waals forces increase with increasing numbers of atoms in a molecule. This is familiarly illustrated in the homologous series of the alkanes, where the C1 alkane, methane, has the lowest boiling point, with boiling points increasing steadily as the molecules get bigger. This is the basis of fractional distillation as a means of separating the mixture of alkanes in crude oil (see CHEMISTRY REVIEW Vol. 28, No. 2, pp. 26–29).
Looking at the molecules produced by the African weaver ant (Figure 10), three of these contain the same functional group, the carbonyl group (–C=O). Their boiling points would be expected to increase in the order hexanal (C6 ) < undecan-3-one (C11) < 2-butyloct-2-enal (C12 ), thus hexanal will be the most volatile of these. The fourth molecule, hexan-1-ol, has the same number of carbon atoms as hexanal, but has stronger intermolecular forces, since the –OH group in hexan-1-ol can participate in hydrogen-bonding, so that its boiling point is higher than that of hexanal.

Talking with chemicals
Insects do not just use chemicals as weapons — they also use them to communicate with each other. These chemicals are referred to as pheromones or semiochemicals. A complex example of this is provided by the defence system of African weaver ant (Oecophylla longinoda) workers. When outsiders enter the weaver ants’ territory, a worker releases a mixture of chemicals from its mandibular gland. Four substances are involved in calling up fellow worker ants, which have sequential effects, depending upon their volatility (see Box 1). The most volatile, hexanal, spreads furthest and is the first one met by other workers in the area. When they detect it, they rush around until they meet the next most volatile, hexan-1-ol, and start to move towards its source, up the concentration gradient. When they meet the third molecule, undecan-3-one, it stimulates them to bite anything they do not recognise (Figure 10). The last of the four chemicals to be encountered, (Z)-2-butyloct-2-enal, makes them even more aggressive towards the intruders, joining with other workers in overpowering the foe. If necessary, they can also spray it with formic acid (methanoic acid).
Insects need to defend themselves, and use a variety of chemicals to achieve this, also employing colour to warn predators of their defence system. The chemistry involved makes a big difference in their small world.
PRACTICE EXAM QUESTIONS
1 Name the two functional groups present in each of the following molecules. (3 marks)
i Cantharidin (Figure 9)
ii Kempane (Figure 6)
iii Trinervi-2β,3α,9α-triol 9-O-acetate (Figure 6)
2 a Which of the four substances in Figure 10 would react with: (3 marks)
i 2,4-dinitrophenylhydrazine
ii ammoniacal silver nitrate
iii sodium metal
b What reagent would you use to convert hexanal into hexan-1-ol? (1 mark)
ChemistryReviewExtras
Check your answers at www.hoddereducation.co.uk/chemistryreviewextras
GLOSSARY
Aposematic Refers to a visual signal, such as warning colours, showing that an organism has dangerous or toxic characteristics.
Basic Describes a substance (base) that acts as an electron pair donor. Bases can neutralise acids.
Denature To break down the regular three-dimensional structure of a protein (e.g. an enzyme) and thus alter its chemical behaviour. When an enzyme changes shape, through heating or chemical reaction, so that it no longer functions, it is said to be denatured.
Homologous series A family of compounds with the same functional group and similar chemical properties. They differ in the number of –CH 2 groups. The members of the series have a general formula (e.g. Cn H2n+2 for alkanes).
Pheromone A chemical secreted and released by an animal for detection and response by another animal, usually of the same species. It is a means of communication. An alarm pheromone is a chemical that an organism releases to warn others of danger.
Semiochemical A chemical used in communication in animals.
van der Waals forces Weak attractive forces between atoms or molecules caused by three factors: permanent dipole– permanent dipole interactions, permanent dipole–induced dipole interactions and instantaneous dipole–induced dipole interactions.
Volatile Describes a substance that vaporises easily.
READ MORE…
Useful articles from past issues of Chemistry review: ‘Bees, honey and venom’, Vol. 28, No. 1, pp. 6–10.
‘Sex and scents in the natural world’ (pheromones), Vol. 6, No. 3, pp. 2–6.
KEY POINTS
■ Insects use chemicals to communicate messages, but also as weapons.
■ These chemical weapons are sprayed or secreted to inflict damage to their predators.
■ The chemicals that are produced can be quickly synthesised via reactions within the insect’s body. Or the substances are stored, ready to be released when required.





