
EXAM LINKS
This article links to the following topics in the AQA, Edexcel, OCR, WJEC, CCEA, SQA and IB Diploma exam specifications:
■ oxidation and reduction (redox reactions)
■ primary, secondary and tertiary alcohols
■ oxidation of alcohols
■ carbonyl compounds
■ green chemistry
■ industrial processes
Chemical reactions that change the oxidation state of organic molecules (Box 1) are crucial to the synthesis of pharmaceuticals, agrochemicals, polymers, fragrances and flavourings. In the pharmaceutical industry, redox reactions are responsible for 15% of reactions used to make medications. As well as coming up with new ways to make bonds, chemists are always looking for methods to make molecules in a way that is more efficient, economical and ‘green’. One method of doing redox reactions, which is becoming increasingly popular, is via electrochemistry.
‘Green’ in the context of the chemical industries means making changes that reduce the negative impact of chemicals on the environment and on health. Twelve principles of green chemistry have been adopted by the chemical industry and academia. These are largely concerned with reducing risks and environmental impacts. One target to lower environmental impact is to reduce the amount of waste produced. This can be achieved by decreasing the amount of organic solvent used, redesigning synthetic routes, managing energy more efficiently and considering how easily something can be recycled. A key area for waste reduction is the toxic by-products from reactions. These chemicals have health risks, so need to be handled and disposed of carefully.
Box 1 Redox reactions
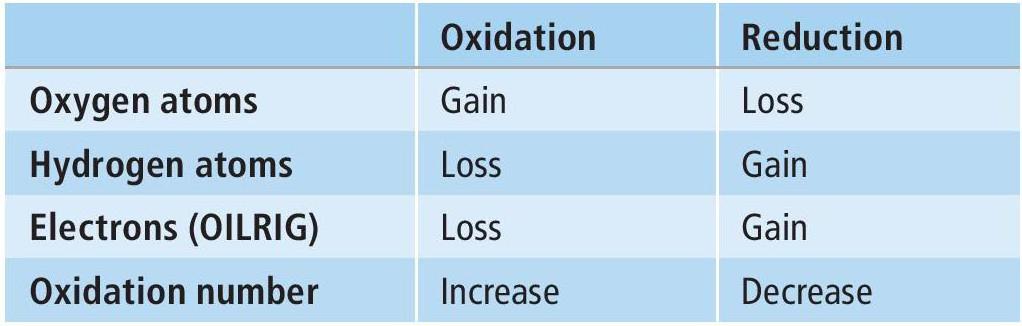
Oxidation and reduction are key chemical conversions in organic synthesis. These processes can be seen in terms of changes in oxygen atoms, hydrogen atoms, electrons and oxidation number (Table 1). As far as the movement of electrons is concerned, use an acronym to help you remember which reaction is which: OILRIG — Oxidation Is Loss, Reduction Is Gain of electrons.
Wasteful reactions
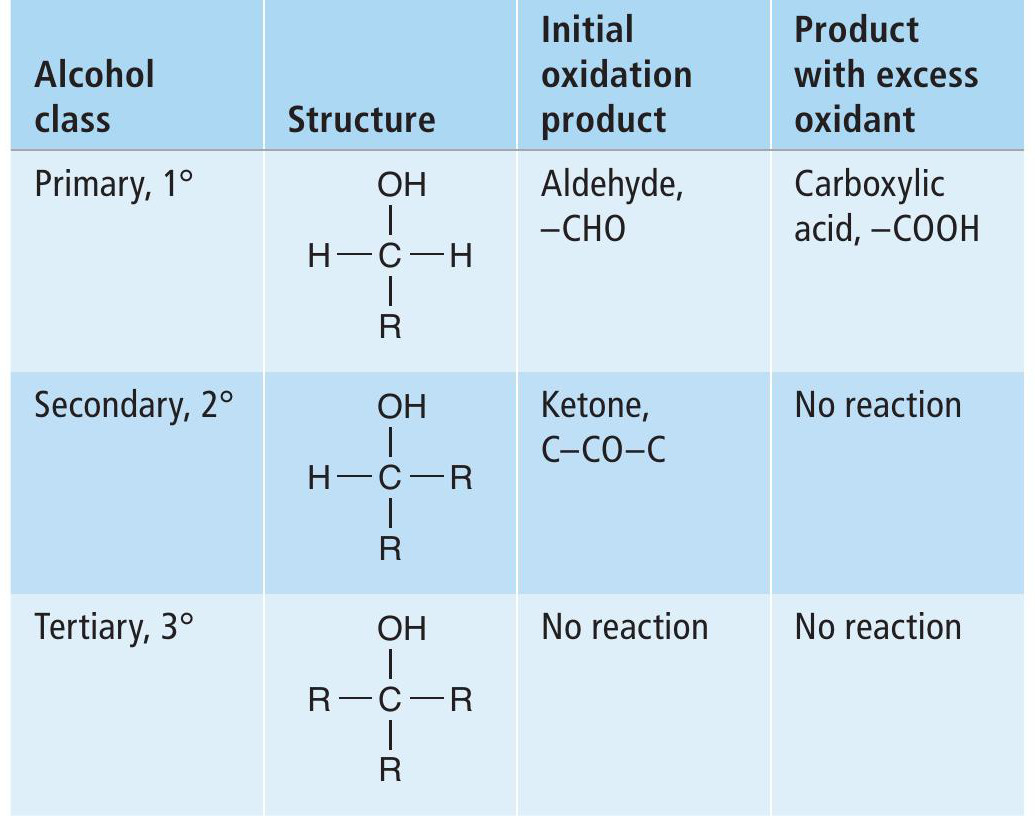
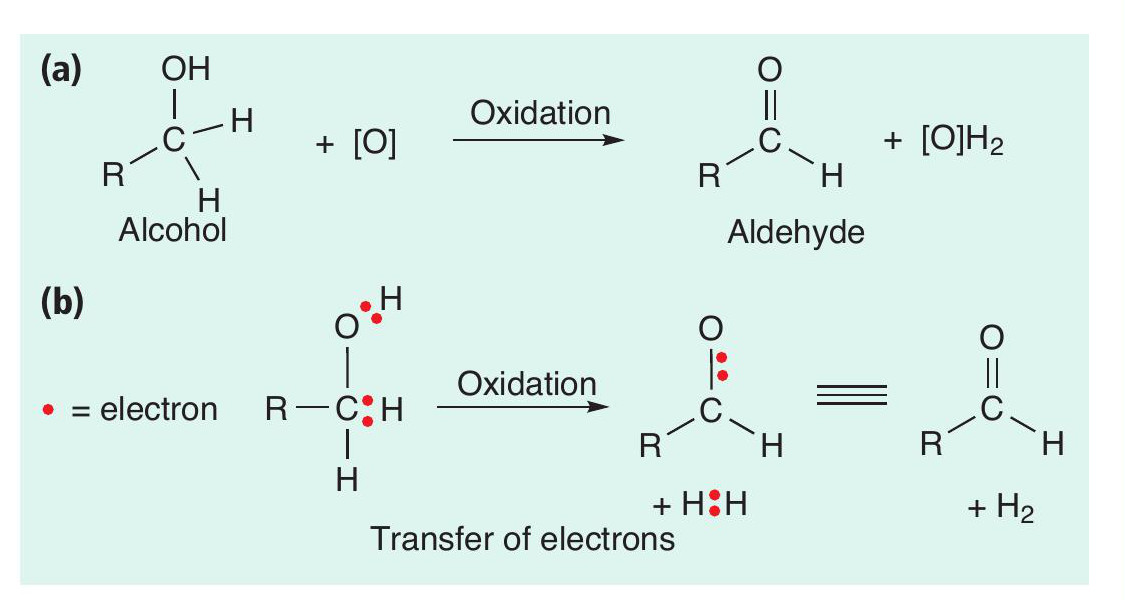
In many syntheses the oxidation of alcohols is a common transformation (Box 2). An example of the oxidation of a primary alcohol to an aldehyde is shown in Figure 1. Overall, the action of an oxidising agent (oxidant) — usually symbolised by [O] — on the alcohol results in the removal of two hydrogen atoms from the alcohol, producing a carbonyl group (C=O). In this process the oxidant is used up and a reduced chemical species of the oxidising agent [O]H2 is formed. One widely used chemical oxidant is potassium dichromate(vi), K 2Cr2O7 (Box 3). However, chromium compounds are highly toxic and can cause cancer, so must be handled with care. A chromium-based oxidation is used for the industrial production of vitamin K, but for every 1kg of product formed, 18 kg of solid chromium waste are produced. To make this process greener, it is crucial to reduce the amount of this toxic waste produced, to minimise the risk to human and animal health and the environment. While many alternative oxidants have been devised over the years, any oxidant used in stoichiometric quantities will create as much waste as it does product.
Box 2 Oxidation of alcohols
Oxidation of alcohols by acidified potassium dichromate(VI) solutions leads to the production of carbonyl compounds if a reaction occurs that does not break a carbon-to-carbon bond. Whether a reaction occurs depends on the class of alcohol. Oxidation of primary, secondary and tertiary alcohols leads to different results (Table 2). Resultant aldehydes can be further oxidised to carboxylic acid.
Electrochemistry
Electrochemistry can be used to generate electrical energy from the spontaneous redox reaction that results when two appropriate metals are submerged in an electrolytic solution. This is called a Galvanic cell and is used in batteries to convert stored chemical energy into electrical energy. Alternatively, electrical energy can be applied to a solution through two electrodes and used to perform a chemical reaction that otherwise would not be spontaneous. This is called an electrolytic cell (see CHEMISTRY REVIEW Vol. 32, No. 2, pp. 16–19) and can be used to perform redox reactions (Figure 2).
As well as thinking about oxidation reactions as a loss of hydrogen atoms, we can also think about them as removing electrons from the starting material (Box 1). In electrochemistry this can be thought of as an electron being directly transferred from the starting material to an electrode. This breaks a covalent bond to a hydrogen atom, with a proton (H+) being formed.
Box 3 Potassium dichromate(VI) as an oxidant
Acidified (excess H+ (aq)) chromate(VI) ions (dichromate ions, Cr2O7 2–(aq)) are a traditional oxidising agent. The potassium ions (K+ (aq)) are spectator ions, that is, they do not take part in the reaction.
Equation 1 is the half-equation for the alcohol (RCH2OH) — the oxidative step:
RCH2OH(l) ➝ RCHO(l) + 2H+(aq) + 2e– (1)
primary alcohol aldehyde
The alcohol has (a) lost hydrogen and (b) lost electrons.
Equation 2 is the half-equation for the chromate(VI) ions — the reductive step:
Cr2O7 2–(aq) + 14H+(aq) + 6e–➝ 2Cr3+(aq) + 7H2O(l) (2)
The chromate(VI) ions have (a) lost oxygen, (b) gained electrons and (c) reduced in oxidation number.
To balance the equations, consider the electrons. Multiply Equation 1 by 3 (so that each equation has 6 electrons).
Then add the two half-equations together:
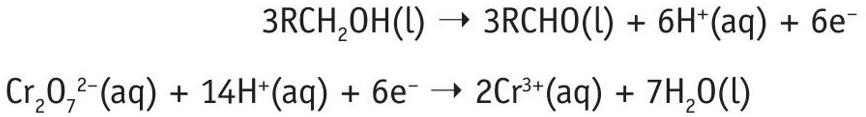
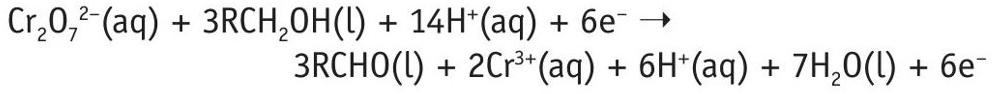
Cancel the electrons and rationalise the hydrogen ions to get a balanced equation:
Cr2O 72–(aq) + 3RCH2OH(l) + 8H+(aq) ➝ 3RCHO(l) + 2Cr3+(aq) + 7H2O(l)
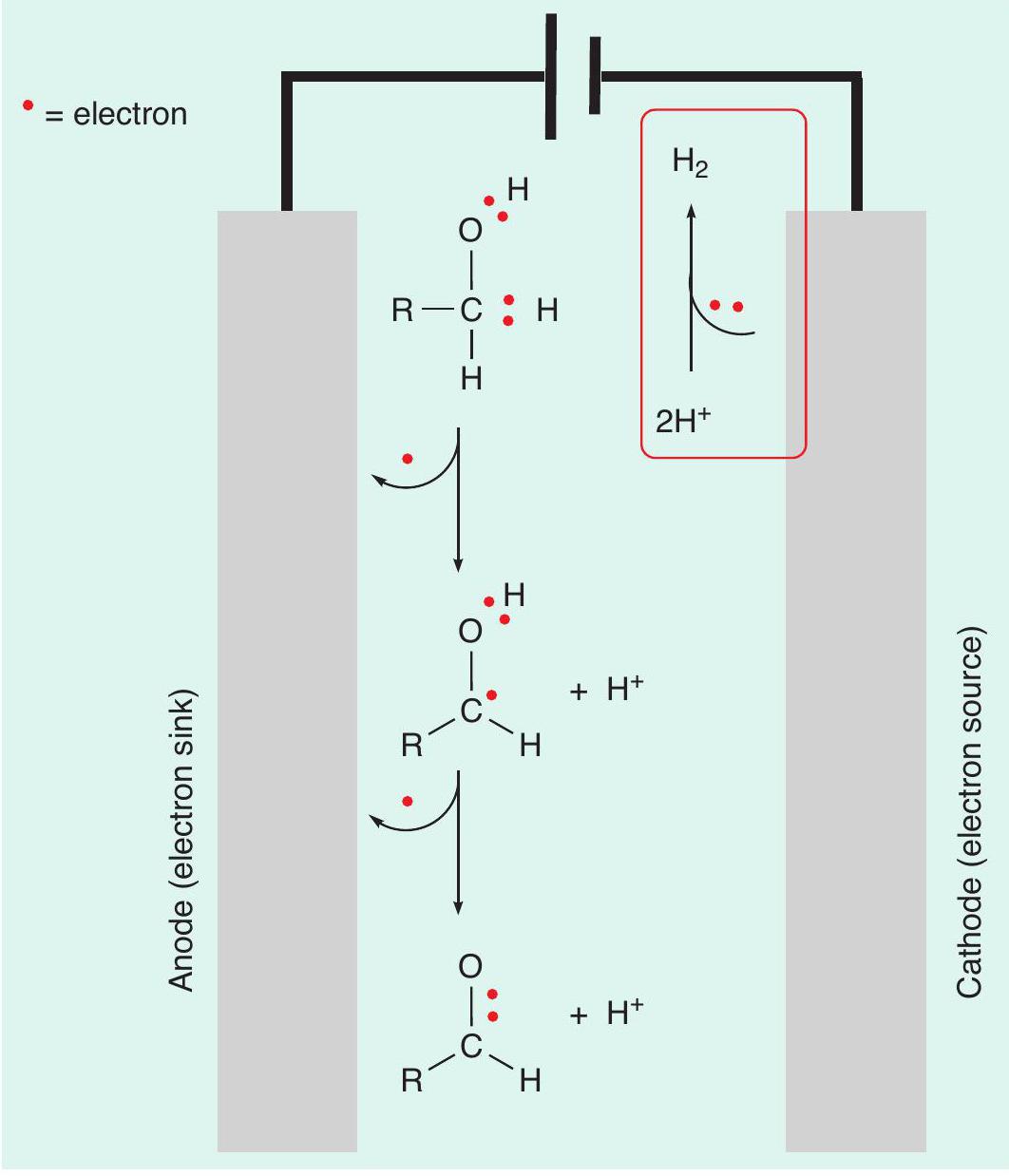
Repeating this process once more removes the second electron necessary for the complete oxidative transformation.
The loss of two protons and two electrons leaves behind two unbonded electrons. These form the pi bond of the desired aldehyde. This can be compared with the use of acidified potassium chromate(vi).
So far, we have completed the oxidative step (removing electrons from the reaction solution) but in the same way that an oxidation reaction by a chemical reagent requires the reagent to be reduced, the electrochemical reaction also requires something to be reduced. Electrons are added to a chemical species in solution. In this case, the electrons can be added to the protons (H+) that have been produced. These protons are reduced to generate hydrogen gas (2H++ 2e–➝ H2). Overall, the starting alcohol has been oxidised to an aldehyde, with only H 2produced as a by-product. So not only has this process avoided the generation of large quantities of toxic chromium waste, but also the hydrogen produced is a useful by-product that can be used as a fuel (CHEMISTRY REVIEW Vol. 31, No. 2, pp. 28–30).
Fine tuning
The oxidation of alcohols to aldehydes is just one reaction that can be carried out using electrochemistry. Research into improving existing transformations through electrochemistry, and coming up with new reactions using electrochemistry, is being developed in academic and industrial laboratories.
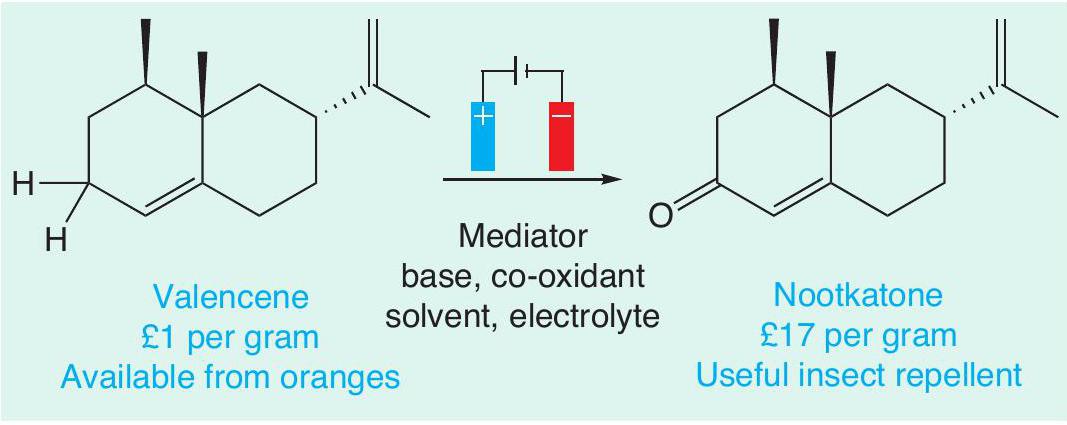
One of the key advantages of electrochemistry is that the conditions (electrolyte, electrode materials and solvent) can be easily fine-tuned to oxidise only a targeted functional group in a molecule that contains many similar groups. This is exceptionally helpful in the synthesis of complex organic molecules, such as pharmaceuticals. This level of control can be hard to achieve with traditional oxidising agents. For example, an electrochemical method was recently developed that allows a remarkably selective oxidation of C–H bonds (Figure 3). These bonds are normally unreactive and breaking them requires reagents based around toxic elements, such as chromium and selenium. Valencene, which can be sustainably sourced from oranges, can easily be turned into nootkatone, which can be used as an insect repellent.

Reductions
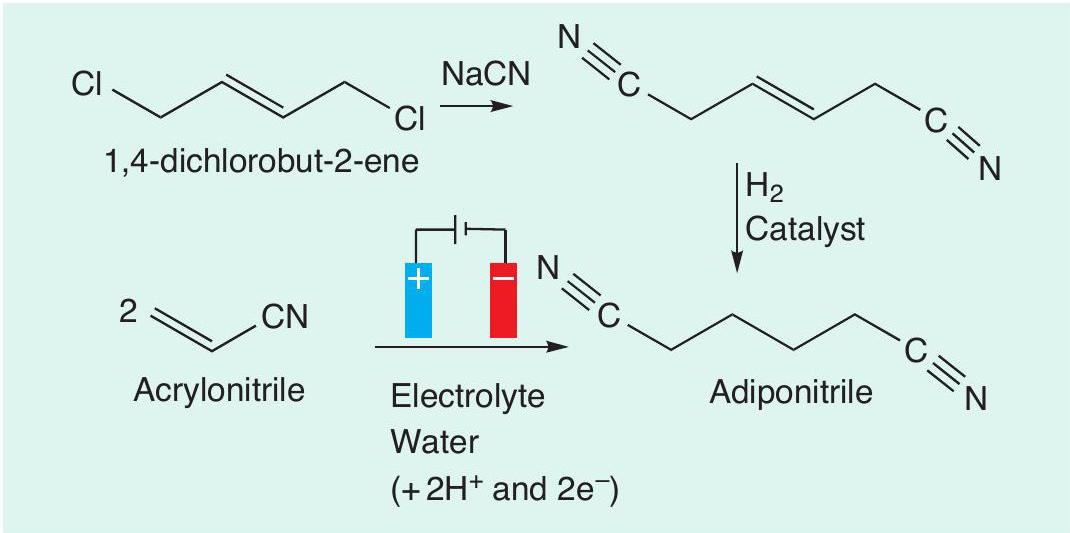
As well as oxidation reactions, reduction reactions are also possible using electrochemistry. An example of this is the synthesis of adiponitrile (hexanedinitrile), which is a crucial precursor for nylon production (CHEMISTRY REVIEW Vol. 20, No. 1, pp. 29–32). One way in which adiponitrile can be produced is from 1,4-dichlorobut-2-ene. The first step of this route is the nucleophilic substitution of chloride with cyanide (CN–) to form a nitrile (–C≡N). This compound can then be hydrogenated to adiponitrile. This route requires the use of sodium cyanide (NaCN), which is highly toxic, and also an expensive catalyst is needed for the hydrogenation step. Instead, an electrochemical method can be used to make adiponitrile in a single step from acrylonitrile (prop-2-enenitrile, Figure 4). This process, called the Baizer process, is used to produce thousands of tonnes of adiponitrile every year. The adiponitrile can then be converted to hexane-1,6-diamine, which is one of the monomers used in the condensation polymerisation process for making one of the nylons.
There is an increasing desire and need to apply green chemistry principles to the manufacture of chemicals that we need. Electrolytic chemistry research and application will be increasingly used in the synthesis of existing and new molecules. Indeed, the method allows some transformations that are only possible through electrochemistry.
PRACTICE EXAM QUESTIONS
1 Draw the skeletal structure of hexane-1,6-diamine. (1 mark)
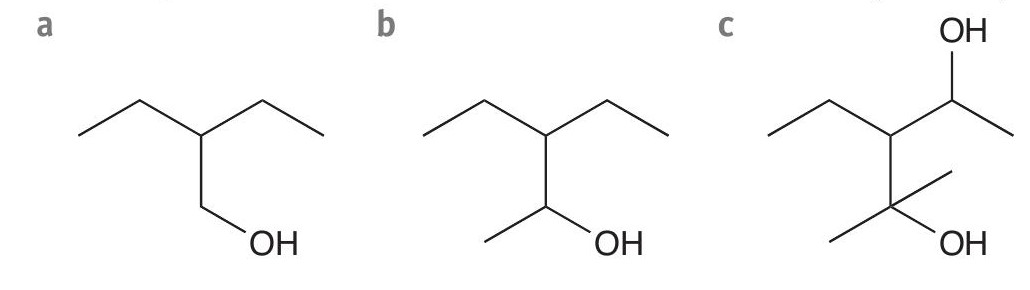
2 What is the final oxidation product for a reaction of acidified potassium dichromate(VI) with each of these compounds? (3 marks)
GLOSSARY
Galvanic cell Also called a voltaic cell, it is a device with two electrodes in an electrolyte (a substance that conducts electricity via the movement of charged ions). The cell produces electricity from chemical reactions that take place at the electrodes.
Half-equation An equation involving electrons that shows the oxidation (or reduction) of a species, without identifying the oxidising (or reducing agent). Half-equations show what is happening to the electrons during a reaction.
Hydrogenation A reaction in which hydrogen is added, causing the hydrogenated substance to be reduced.
Nucleophilic substitution A nucleophile is an electron-rich species, such as a negative ion. When a nucleophile attacks an electron-deficient portion of a molecule and replaces an atom or group of atoms, the reaction is described as nucleophilic substitution (see CHEMISTRY REVIEW Vol. 30, No. 4, pp. 7–9).
Oxidation number (or oxidation state) It is equal to the difference between the number of electrons associated with an element in a compound and in the element itself. It is a measure of the extent of oxidation of a compound, i.e. how electron-deficient it is (see CHEMISTRY REVIEW Vol. 26, No. 4, p. 12).
Pi bond (π bond) A bond between two atoms arising from the sideways overlapping of p-orbitals (an orbital is a region around an atom where an electron has a high probability of being found).
Redox reaction Oxidation-reduction reaction where electrons are lost by one species (oxidation) and gained by another species (reduction).
Stoichiometric Describes chemical reactions in which the reactants combine in simple whole-number ratios.
Vitamin K A group of chemically similar fat-soluble compounds (obtained through the diet) that are essential for the liver to be able to produce various blood coagulation and clotting factors.
ChemistryReviewExtras
Check your answers at www.hoddereducation.co.uk/chemistryreviewextras





